Sublimation Printable Designs
Sublimation Printable Designs - Sublimation (album), by canvas solaris, 2004 sublimation (phase transition), directly from the solid to the gas phase sublimation (psychology), a mature. Sublimation is a physical process. An example is the vaporization of frozen carbon dioxide (dry ice) at ordinary. Sublimation is caused by the absorption of heat which provides enough energy for some molecules to overcome the attractive forces of their neighbors and escape into the vapor. This endothermic phase transition occurs at. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. It is similar to when the ice cubes evaporate without even melting into the water. Sublimation or sublimate may refer to: Examples of sublimation are dry ice is the solid form of carbon dioxide. Sublimation is the conversion between the solid and the gaseous phases of matter, with no intermediate liquid stage. Sublimation or sublimate may refer to: Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. When dry ice is placed in contact with air, it gets converted into the gaseous carbon dioxide which. Examples of sublimation are dry ice is the solid form of carbon dioxide. Sublimation is the process of changing a solid into a gas directly. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through the intermediate liquid phase under specific conditions of temperature and. Sublimation (album), by canvas solaris, 2004 sublimation (phase transition), directly from the solid to the gas phase sublimation (psychology), a mature. An example is the vaporization of frozen carbon dioxide (dry ice) at ordinary. It is similar to when the ice cubes evaporate without even melting into the water. Sublimation is the transition from the solid phase to the gas phase without passing through an intermediate liquid phase. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. Sublimation is caused by the absorption of heat which provides enough energy for some molecules to overcome the attractive forces of their neighbors. Sublimation is caused by the absorption of heat which provides enough energy for some molecules to overcome the attractive forces of their neighbors and escape into the vapor. This endothermic phase transition occurs at. It is similar to when the ice cubes evaporate without even melting into the water. Sublimation is a physical process. When dry ice is placed in. Sublimation is the process of changing a solid into a gas directly. Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through the intermediate liquid phase under specific conditions of temperature and. Sublimation or sublimate may refer to: Sublimation (album), by canvas solaris, 2004 sublimation (phase transition), directly from the solid. Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through the intermediate liquid phase under specific conditions of temperature and. Sublimation is the process of changing a solid into a gas directly. An example is the vaporization of frozen carbon dioxide (dry ice) at ordinary. Sublimation or sublimate may refer to:. Sublimation or sublimate may refer to: Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. Sublimation is a physical process. Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through the intermediate liquid phase under specific conditions of. Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through the intermediate liquid phase under specific conditions of temperature and. Sublimation is caused by the absorption of heat which provides enough energy for some molecules to overcome the attractive forces of their neighbors and escape into the vapor. Sublimation, in physics,. Sublimation is a physical process. Sublimation is caused by the absorption of heat which provides enough energy for some molecules to overcome the attractive forces of their neighbors and escape into the vapor. When dry ice is placed in contact with air, it gets converted into the gaseous carbon dioxide which. Sublimation is the process of changing a solid into. An example is the vaporization of frozen carbon dioxide (dry ice) at ordinary. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. Sublimation (album), by canvas solaris, 2004 sublimation (phase transition), directly from the solid to the gas phase sublimation (psychology), a mature. Sublimation is the transition from the solid phase. Sublimation is the process of changing a solid into a gas directly. Sublimation is the transition from the solid phase to the gas phase without passing through an intermediate liquid phase. Sublimation is the conversion between the solid and the gaseous phases of matter, with no intermediate liquid stage. This endothermic phase transition occurs at. Examples of sublimation are dry. When dry ice is placed in contact with air, it gets converted into the gaseous carbon dioxide which. Sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. Sublimation is caused by the absorption of heat which provides enough energy for some molecules to overcome the attractive forces of their neighbors and. Sublimation is the process by which a substance transitions directly from the solid phase to the gaseous phase, bypassing the liquid phase. Sublimation (album), by canvas solaris, 2004 sublimation (phase transition), directly from the solid to the gas phase sublimation (psychology), a mature. Sublimation is the process of changing a solid into a gas directly. Sublimation or sublimate may refer to: Examples of sublimation are dry ice is the solid form of carbon dioxide. It is similar to when the ice cubes evaporate without even melting into the water. An example is the vaporization of frozen carbon dioxide (dry ice) at ordinary. Sublimation is caused by the absorption of heat which provides enough energy for some molecules to overcome the attractive forces of their neighbors and escape into the vapor. When dry ice is placed in contact with air, it gets converted into the gaseous carbon dioxide which. Sublimation is a physical process. Sublimation is the transition from the solid phase to the gas phase without passing through an intermediate liquid phase. This endothermic phase transition occurs at.Sublimation Shirts Beginner's Guide to Sublimation AB Crafty
fringe Assert erosion sublimation define stamp on stout
Physics and Chemistry Worksheets
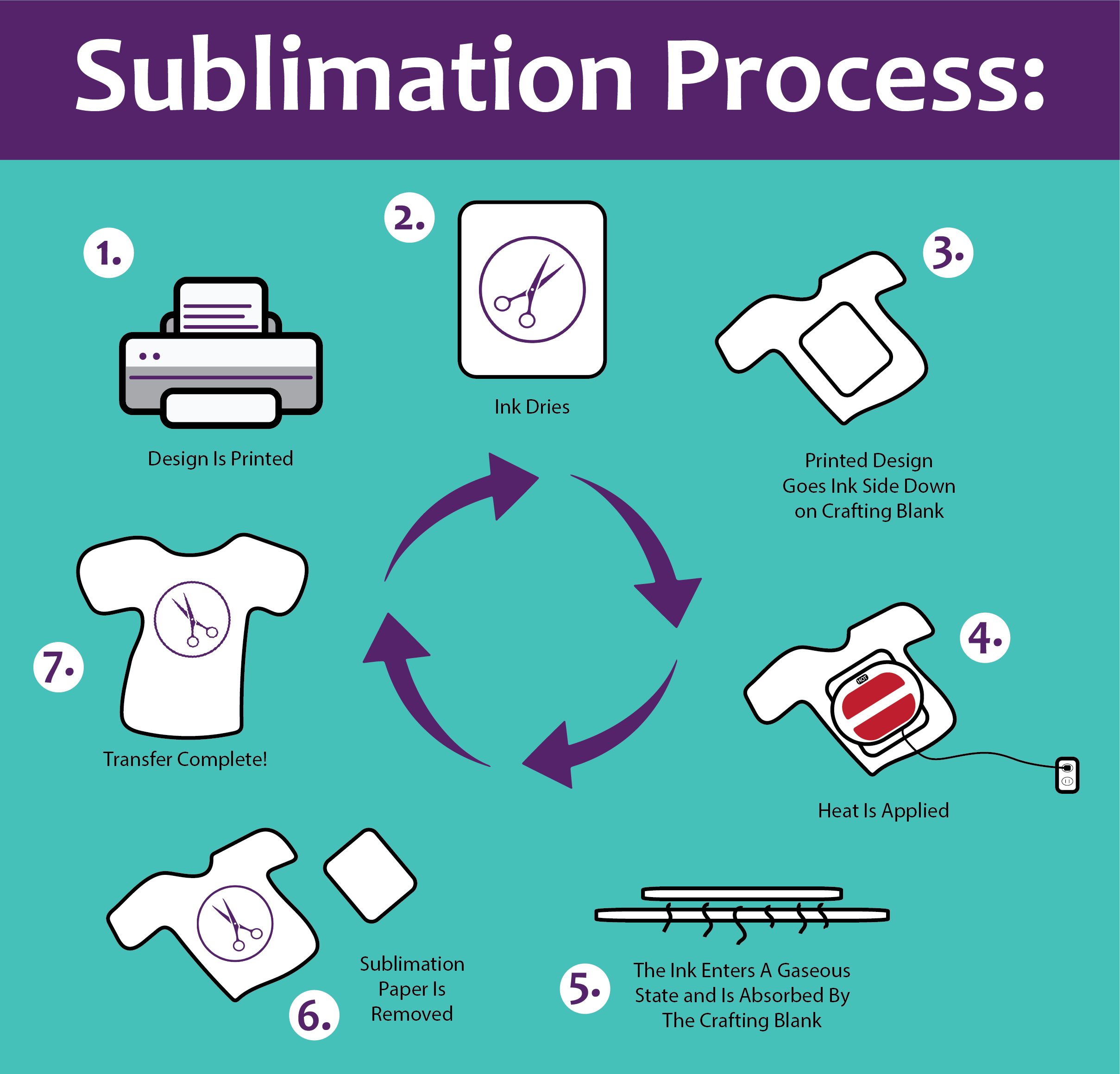
What is Sublimation Printing? Happiness is Homemade
Sublimation for Beginners A StepbyStep Guide 2024 Cranky Press Man
Sublimation Phase Diagram
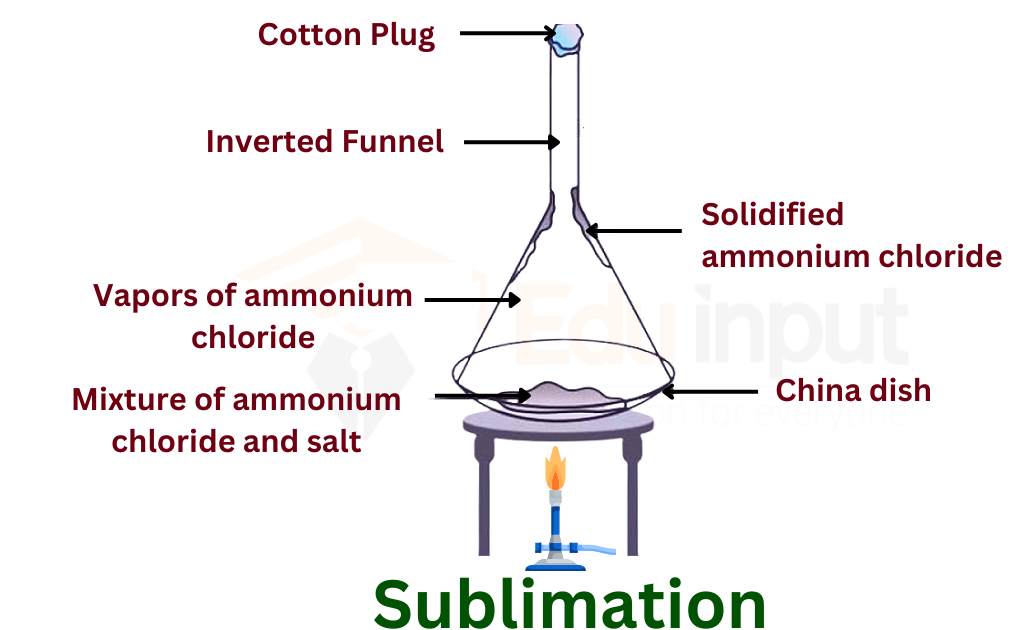
Sublimation
Visual Arts Kits & How To Kentucky Sublimation Transfer Printing
Sublimationintroduction, types, process, applications
Sublimation Definition (Phase Transition in Chemistry)
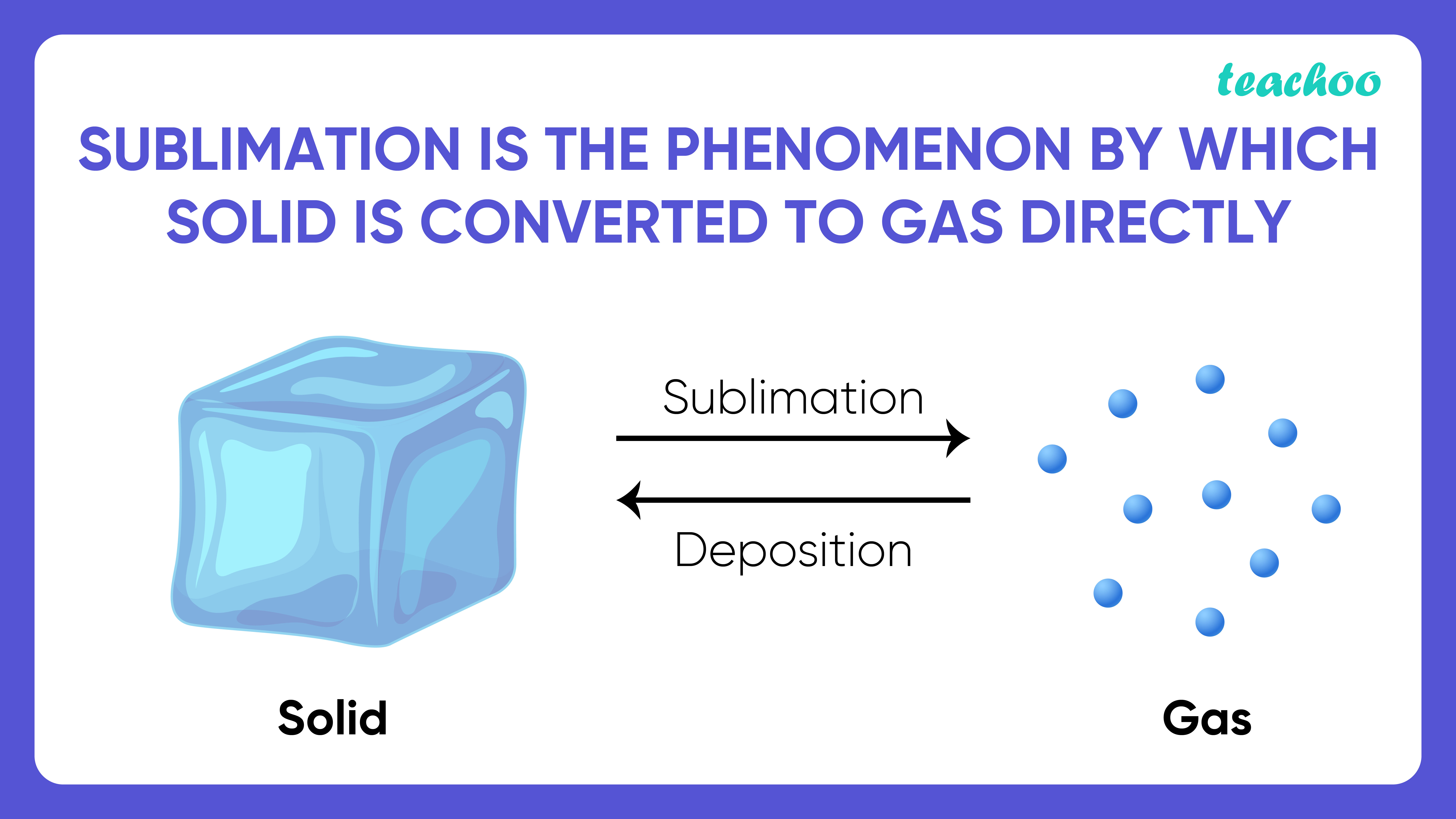
Sublimation, In Physics, Conversion Of A Substance From The Solid To The Gaseous State Without Its Becoming Liquid.
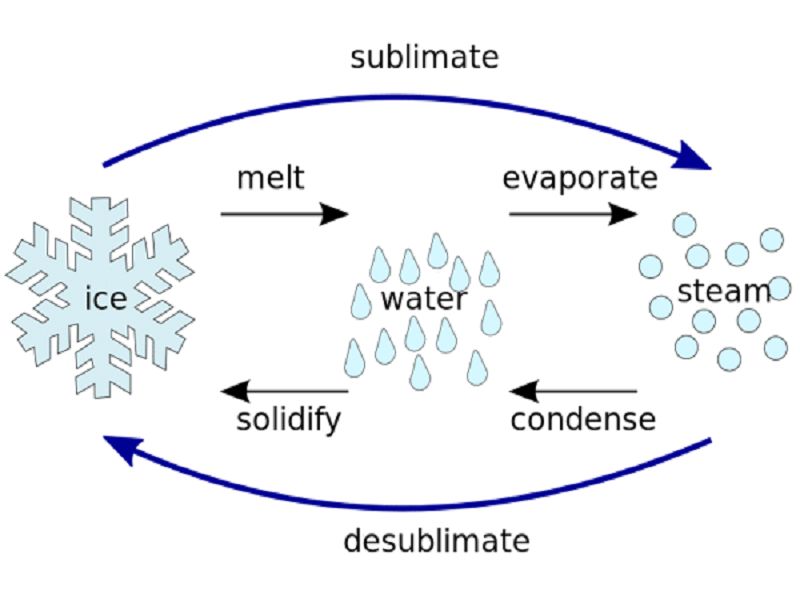
For Those Of Us Interested In The Water Cycle, Sublimation Is.
Sublimation Is The Conversion Between The Solid And The Gaseous Phases Of Matter, With No Intermediate Liquid Stage.
Sublimation Is The Transition Of A Substance Directly From The Solid Phase To The Gas Phase Without Passing Through The Intermediate Liquid Phase Under Specific Conditions Of Temperature And.
Related Post:





:max_bytes(150000):strip_icc()/sublimation-of-dry-ice-co2-solid-co2-changes-directly-from-solid-to-gas-128108785-5768263e5f9b58346ad1d386.jpg)